
Negotiating the Relationship Between Addiction, Ethics, and
Brain Science
Daniel Z. Buchman
1,*
, Wayne Skinner
2,3
, and Judy Illes
1,4
1
National Core for Neuroethics, The University of British Columbia, Vancouver, BC, Canada
2
Centre for Addiction and Mental Health, Toronto, ON, Canada
3
Department of Psychiatry, The University of Toronto, Toronto, ON, Canada
4
Division of Neurology, University of British Columbia, Vancouver, BC, Canada
Abstract
Advances in neuroscience are changing how mental health issues such as addiction are understood
and addressed as a brain disease. Although a brain disease model legitimizes addiction as a
medical condition, it promotes neuro-essentialist thinking, categorical ideas of responsibility and
free choice, and undermines the complexity involved in its emergence. We propose a
‘biopsychosocial systems’ model where psycho-social factors complement and interact with
neurogenetics. A systems approach addresses the complexity of addiction and approaches free
choice and moral responsibility within the biological, lived experience and socio-historical context
of the individual. We examine heroin-assisted treatment as an applied case example within our
framework. We conclude with a discussion of the model and its implications for drug policy,
research, addiction health care systems and delivery, and treatment of substance use problems.
Keywords
mental health; neuroethics; public health; sociology
Brain Biology and Addiction
Recent advances in neuroscience provide compelling evidence to support a medical
perspective of problematic substance use and addiction (Dackis and O’Brien 2005). Despite
these developments, the science is still in its early stages, and theories about how addiction
emerges are neither universally accepted nor completely understood. Current ethical and
legal debates in addiction draw upon new knowledge about the biological and neurological
modification of the brain (Ashcroft, Campbell, and Capps 2007). Clinically, substance use
disorders
1
are characterized by compulsive engagement or impaired control over behaviour,
evidence of both tolerance and withdrawal, relapse despite continued harm, impairments in
social and occupational functioning, and irritability or intense cravings when a particular
substance is not directly available (American Psychiatric Association 2004).
Functional brain changes occur in response to stress and other stimuli in people who have
substance use problems. Recent neuroimaging studies suggest that people living with a drug
*
Correspondence Daniel Z. Buchman, National Core for Neuroethics, The University of British Columbia, 2211 Wesbrook Mall,
Koerner S124, Vancouver, BC V6T 2B5 CANADA, Tel: 604.822.0748, Fax: 604.827.5229, [email protected].
1
The DSM-IV-TR differentiates between Substance Dependence and Substance Abuse. In this paper we use the term “substance use
disorder” or “addiction” to refer to both the complex nature of severe substance dependence and substance abuse.
NIH Public Access
Author Manuscript
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
Published in final edited form as:
AJOB Neurosci
. 2010 January ; 1(1): 36–45.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

addiction have considerable decreases in dopamine D2 receptors and in dopamine release
(Volkow, Fowler, Wang, Baler, and Telang 2009), which may contribute to both the
rewarding properties of substances and difficulties in abstaining despite adverse
consequences. Brain areas such as the prefrontal cortex have been identified as being
directly involved in assessing the reward potential of decision-making (Bechara and
Damasio 2002; Dom, Sabbe, Hulstijn, and Van Den Brink 2005), and vulnerability to
relapse. Abnormal hippocampus and anterior cingulate functioning is associated with
challenges in the ability to cope with stress, in addition to problems in cognition (Kaufman,
Ross, Stein, and Garavan 2003) such as salience, inhibitory control, motivation, memory,
and learning (Hyman 2005). Increased activation of the dorsal striatum has been linked with
a vulnerability to strong cravings (Sinha and Li 2007). In addition to the recent studies that
are investigating neurogenetic contributions to a vulnerability to addiction (Uhl, Drgon,
Johnson, and Liu 2009), arguments have been made for a somatic marker theory of
addiction (Verdejo-Garcia and Bechara 2009).
A neurobiological perspective has the potential to provide many benefits to people with
addiction in terms of psychopharmacological and other treatment options. However purely
reductive, neurobiological explanations of addiction occlude a comprehensive understanding
of the added influence of psychological, social, political, and other factors. The view of
addiction as primarily a brain disease (Leshner 1997) disregards the extensive body of
research that suggests neurogenetic explanations of mental illness contribute to negative
perceptions towards people with mental illness and substance use problems (Dietrich,
Matschinger, and Angermeyer 2006; Read, 2007; Read, Haslam, Sayce, and Davies 2006).
This view is problematic as individuals living with an addiction are highly stigmatized. The
brain disease model further implies simplistic categorical ideas of responsibility, namely that
addicted individuals are unable to exercise any degree of control over their substance use
(Caplan 2006, 2008). This kind of “neuro-essentialism” (Racine, Bar-Ilan, and Illes 2005)
may bring about unintentional consequences on a person’s sense of identity, responsibility,
notions of agency and autonomy, illness, and treatment preference.
We argue therefore for a biopsychosocial systems model of, and approach to, addiction in
which psychological and sociological factors complement and are in a dynamic interplay
with neurobiological and genetic factors. As Hyman (2007) has written, “neuroscience does
not obviate the need for social and psychological level explanations intervening between the
levels of cells, synapses, and circuits and that of ethical judgments” (p.8). Since the so-
called brain disease model of addiction does not resolve the volitional nature of substance
use completely, a biopsychosocial systems approach attempts to contextualize the
individual, thus providing a model to better understand both responsibility and self in
addiction.
This paper builds on the conceptual foundations of Hyman’s (2007) contribution on
addiction and voluntary control, and extends the thinking to include perspectives that
include, but also go beyond, neuroscience.
Psycho-Social Systems
Psycho-social systems are concrete entities or groups whose members act in relation to each
other, such as families, religious organizations, and political parties (Bunge 2004). Social
processes in addiction are investigated by examining social categories such as networks,
groups, organizations and subcultures that alone cannot be explained by neurobiology.
Addiction consists of interacting biological and psychosocial mechanisms because the
mechanism (e.g., the behaviour) contributing to addiction involves action within a social
system. The larger societal structure either restricts or enhances interactions between agents
Buchman et al. Page 2
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

in a social system (Bunge 1997). Such actions require explanations at both the systemic and
individual levels.
Every learned action, whether pro-social or anti-social, may be prompted by social
conditions such as a lack of resources, conflict, social norms, peer pressure, an underlying
drive (e.g., hunger, sex, craving), or a combination of these factors (Bunge 1997).
Addiction-related behaviours affect the health of both individuals and communities, either
protectively or harmfully. The behaviours influence the extent an individual is able to
mobilize and access resources to achieve goals and adapt to adverse situations (Raphael
2004). For example, an individual’s socioeconomic status is correlated with increased
negative consequences from substance use, such as increased sharing of used injecting
equipment and higher prevalence rates of Human Immunodeficiency Virus (HIV) and
hepatitis C (Strike, Myers, and Millson 2004).
There are several processes that actively contribute to substance use with inputs and outputs
on biological and psycho-social levels. One example is drug craving that may be
experienced as strong, intense urges for immediate gratification that may impair rational
thought about future planning (Elster and Skog 1999). Cravings can be cue-elicited by
environmental stimuli (Childress, Mozely, McElgin et al. 1999; Loewenstein 2000), and
continued exposures to environmental stimuli may instigate a perpetual cycle of cravings
and possibly irreversible brain changes that can occur long after an individual has become
abstinent. Factors such as drug availability within the environment can increase craving and
consequently the vulnerability for relapse (Weiss 2005). Recent research has suggested that
enriched environments produce long-term neural modifications that decrease neural
sensitivity to morphine-induced reward (Xu, Hou, Gao, He, and Zhang 2007). Accordingly,
the social environment can increase the frequency of cravings, which may contribute to
increased drug consumption, and thus increase the probability that affected individuals will
participate in a series of habituated behaviours that facilitate using (Levy 2007b).
Rates of substance use and dependence vary across, and even within, cultural and social
groups (Wallace 1999; Wallace, Bachman, O’Malley et al. 2002). Factors such as
availability and peer modeling account for the inter- and intra-group disparities (Thomas
2007). These factors may indicate a certain level of group risk for problematic substance
use, but cannot verify either the likelihood of substance use occurring within the group or
which individuals within the group are more likely to be affected. These factors are not
inherent in the composition of the social structure, are neither stable nor persistent, but are
governed by the social values and norms of that social system or group (Bunge 2003).
Social norms regulate behaviour and may act as informal mechanisms of social control.
Social groups construct norms that affect individual behaviour, prevalence, and substance
use patterns. Group membership in which substance use is socially acceptable, encouraged,
or perhaps coerced is significantly associated with patterns of use (Lauer and Lauer 2002).
Group norms around social acceptance of substance use dictate variance in consumption
rates among diverse ethnic and cultural groups (Caetano and Clark 1998). It is these
systemic features that give individuals, in part, motives for action.
Both social norms and laws influence attitudes, perceptions, and beliefs of the effects of
substances and considerably affect consumption rates (Babor, Caetano, Casswell et al. 2003;
Hawkins, Catalano, and Miller 1992). Proponents of a ‘war on drugs’, for example, believe
that laws and policies that are lenient towards substance use are linked with greater
prevalence of use and criminal activity. However, research findings have not confirmed this
claim. In one study comparing cannabis use in San Francisco (where cannabis is
criminalized) and Amsterdam (de facto decriminalization), there was no evidence to support
Buchman et al. Page 3
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

claims that criminalization laws reduce use or that decriminalization increases use. In fact,
San Francisco reported a higher cannabis use rate than Amsterdam (Reinarman, Cohen and
Kaal 2004).
Shifts in norms and laws can propel changes in behaviours associated with substance use
and the prevalence of substance misuse (Hawkins et al. 1992). An example of a profound
shift that has occurred since the latter part of the 20th century is tobacco use and a dramatic
decline in smoking rates (Health Canada 2007). Progress in the understanding of the
pharmacokinetics and pharmacodynamics of nicotine addiction has led to interventions that
have helped many individuals curb smoking behaviour. However, the full understanding of
these normative shifts includes not just the development of medical interventions for
smoking cessation, but also the powerful social and public health efforts to destabilize
smoking behaviour. Therefore, smoking has become less acceptable as a normative method
of social interaction.
Neuroethics and the Brain Disease Model
Long-standing debates concerning the moral status of addiction have arisen from one of two
perspectives: either addiction is a disease of the brain, or addiction is a matter of weak will.
The former absolves responsibility and the latter condemns the person, and thus
distinguishes between deontological and utilitarian positions. Supporters of the brain disease
model cite recent neuroscience research wherein addiction is ontologically reducible to the
level of brain cells (Dackis and O’Brien 2005; Volkow and Li 2005). The claim is that a
brain disease paradigm will decrease stigma associated with addiction, which should
increase access to the health care system. Those inclined to the moral stance see drug use as
an intentional, often criminal, act: the “person, not his autonomous brain, is the instigator of
relapse and the agent of recovery” (Satel 1999, p.861). Other authors take a somewhat
different approach, and conceptualize addiction as a “pleasure-oriented desire” (Foddy and
Savulescu 2006, 2007), skeptical that the continued medicalization of addiction will obviate
all responsibility for behaviours associated with drug use.
Substance Use, Autonomy and Decision-making
Discussions on the relationship between autonomy and decision-making capacity in addicted
persons have received much attention recently in the neuroscience, law, and bioethics
literature (Andreou 2008; Burns and Bechara 2007; Caplan 2006, 2008; Carter and Hall
2008a, 2008b; Hall, Capps, and Carter 2008; Levy 2006, 2007a; Morse 2007). These
writings have raised questions about the autonomy of people with addictions, their ability to
make a free, un-coerced choice, and the extent to which they possess mental competence to
consent to research (Carter and Hall 2008a, 2008b; Charland 2002, 2003; Dehue 2002; Hall,
Carter and Morely 2003a, 2003b). The view that people with addictions lack decision-
making capacity is supported by research in both addiction neuroscience and the
neuroscience of decision-making. For example, in non-addicted persons, studies have
demonstrated that unconscious brain activation directs behaviour and, consequently, action
may not be consciously controlled (Haggard 2008; Soon, Brass, Heinze, and Haynes 2008;
Wegner 2002). This view, effectively, undermines intentional self-determination. For
addiction, a causal neuroscientific model reinforces notions of a diseased brain. The model
suggests that substance use hijacks voluntary brain mechanisms and renders individuals
incapable of making rational decisions (Miller and Carroll 2006). These perspectives imply
a causal neurophilosophical model of decision-making and action, one that has been
critiqued by Gillett (2009). He describes the following trajectory of S_ BE1 _ BE2 _
Output, where:
• “S is the stimulus conditions provoking a decision or action
Buchman et al. Page 4
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

• BE1 is the unconscious brain events preceding an action/decision
• BE2 is the conscious brain event (complex) or intention causing an action/decision
• Output is a bodily movement or a decision” (p. 332).
Gilllett argues that the causal model is based on a faulty account of human autonomy and
consciousness and is scientifically and conceptually questionable. Gillett challenges the
neurophilosophical model of human decision-making, which, as he has previously argued
(2008a), emphasizes selfishness, and “constricts the scope of reason so that it is subject to
any desire or disposition that one happens to endorse at the time one acts” (p. 1215). Gillett
criticizes theories of decision-making that conceptualize choice as autonomous phenomenon
only if inner mental states or networks cause it.
While making a decision is itself a mental act, a mental act or event does not cause
behaviour alone, but is one part of the complex process between neuronal firing and action.
Once an intention has been formed for example, to use substances one is aware of the
intention, though intention itself does not sufficiently cause the individual to seek out or use
drugs. Other factors are at play. From a neuroscience perspective, it is difficult to see such
actions as completely free, particularly when explanations of natural phenomena are
understood as causally ordered. The notion of free choice becomes particularly troublesome
due to the conscious experience of acting freely. As Searle (2004) argues, “there is a striking
difference between the passive character of perceptual consciousness and the active
character of what we might call ‘volitional consciousness’“ (41).
Action, subjective experience of action, and consequently responsibility for action is
mediated by many factors, including psychological phenomenon such as an individual’s
emotional processes. As a point of illustration, Damasio’s (1994) somatic marker hypothesis
(SMH) provides a helpful perspective on integrating the neuropsychological domain of
decision-making and human interaction with the social environment. The SMH proposes a
mechanism where emotion guides or significantly influences behaviour, particularly
decision-making. Somatic markers are acquired by experience and are under control of a
neural “internal preference system [which] is inherently biased to avoid pain, seek potential
pleasure, and is probably pretuned for achieving these goals in social situations” (Damasio
1994, 179). The brain responds to particular social cues that may provide instant pleasure, or
regulate biological homeostasis, such as relief from withdrawal (Li and Sinha 2008). Brain
systems that moderate feeling, memory, cognition, and engage the individual with the world
influence the decision to consume or not consume a drug, or participate in a specific
behaviour or series of actions. Accordingly, this cybernetic brain-environment interaction
may trigger strong somatic signals such as desire, urge and anticipation (Verdejo-Garcia and
Bechara 2009). In effect, this process may limit autonomy as it allows for “preference
reversals” (Levy 2007a) to occur in situations where an individual would rather not use.
The degrees in which self-control is exerted, free choice is realized and desired outcomes
achieved are dependent on these complex interacting biopsychosocial systems. Many post-
modern theorists such as Christman (2004) have challenged the original Kantian privileging
and definition of autonomy. One claim is based on the fact that decisional autonomy, or
rationality, is not the most valuable human characteristic, and the individual-as-independent
does not adequately characterize human beings (Russell 2009). Accordingly, the matrix of a
person’s socio-historical context, life narrative, genetics, and relationships with others
influence intention, decision, and action, and thus shape the brain. Autonomy, therefore, is
not adequately defined just by the events in the brain or the “quality” of the decision being
made. As Gillett (2009) remarks, “a decision is…not a circumscribed event in neuro-time
that could be thought of as an output, and an intention is not a causal event preceding that
output, but both are much more holistically interwoven with the lived and experienced fabric
Buchman et al. Page 5
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

of one’s life” (p. 333). Many individuals who have serious addictions live in impoverished
environments without suitable resources or opportunities. Thus it is the limited option for
choice that is one prevailing variable, not only the reduced ability to choose alternatively.
An individual living with an addiction is in-the-world-with-others and thus acts as a being-
among-others, such that the individual’s decisions and complex engagement with the world
may not be as automatic as the neurophilosophical model may suggest (Gillett 2008a,
2008b, 2009). For that reason, individuals who live with an addiction may not completely be
enslaved or forced by their brain in the way in which, as Levy (2007a) has previously
deferred to Aristotle (1999), “a wind or people have [an agent] in their control were to carry
him off” (p.30). Given the spectrum nature of substance use problems, decision-making
capacity is therefore neither completely present nor absent, but may be, at some times in
certain contexts, weakened. One area in particular in which these neuroethics notions of
addiction may have significant impact is in the clinical setting.
Addiction Neuroethics in the Clinical Context
A future application of clinical neuroscience may allow for more precise prediction of a
neurogenetic vulnerability to addiction, lead to better understanding of pharmacokinetics
and pharmacodynamics of drug use, and to bring greater precision to diagnosis than is
currently possible. Realizing a neurobiological or genetic susceptibility to addiction could
empower life planning and the avoidance of high-risk scenarios. Individuals involved in
treatment could learn effective coping strategies, modify proximal environmental triggers,
and achieve other social goals. Yet when neurogenetic attributions are made entirely
irrespective of their social context, individuals with mental health problems are viewed as
less responsible (Mehta and Farina 1997), and the individuals themselves may perceive a
limited control over their actions (Shiloh, Rashuk-Rosenthal, and Benyamini 2002). These
perceptions may greatly affect addiction recovery rates (Godin and Kok 1996). As Hall and
colleagues (2003a) remark, “A ‘disease’ that can be ‘seen’ in the many-hued splendor of a
PET scan carries more conviction than one justified by the possibly exculpatory self-reports
of individuals who claim to be unable to control their drug use” (p.1485).
When neurogenetic attributions are presented in the clinic, pharmacological treatments are
often believed to be a more effective option over psychotherapy (Phelan, Yang, and Cruz-
Rojas 2006). This attribution could sway those who assign the cause of their addiction to be
exclusively neurological or genetically based, and not necessarily evaluate the risks and
benefits of pharmacotherapy, psychotherapy, or receiving both as combination. These causal
neurogenetic attributions have led some authors to advocate for involuntary treatment in
addiction, arguing that, paradoxically, autonomy must be denied, “in order to create it”
(Caplan 2008). In these extreme examples, a Ulysses contract (Andreou 2008) may be more
respectful of autonomy, and relevant harm reduction approaches in the clinical setting may
help empower a person to be more autonomous in their treatment decisions, pursue what is
meaningful to him or her, and accept the accompanying responsibilities (Buchman and
Russell 2009).
Notions of a pathologized self, deeply enmeshed with personal identity, may lead an
individual to internally negotiate a relationship between the self and the brain (Dumit 2003).
It may further challenge understandings of “accepted” identities, such as health seeking and
rational, as opposed to “contested” identities, such as addict, intoxicated, and at-risk (Fry
2008). The latter may compromise an individual’s sense and experience of free will, being-
in-the-world, perceptions of personal responsibility, and view abnormalities in dopamine
pathways as fatalistic.
Buchman et al. Page 6
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

Guiding an individual’s behaviour are brain processes, somatic mechanisms, the ethical
rules and norms that govern society, and the nature of the interaction. The complex
combination of biological, psycho-social and systemic factors may explain why it is so
difficult for some individuals to refuse drugs in the face of increasingly negative
consequences. An underlying feature of these interacting systems is the human subjective
experience of free voluntary actions, which problematizes laws within the natural world that
every event has a cause with causally sufficient explanations.
Biopsychosocial Systems Approach
The biopsychosocial model of addiction (Figure 1) posits that intersecting biological,
psycho-social and systemic properties are fundamental features of health and illness. The
model includes the way in which macro factors inform and shape micro systems and brings
biological, psychological and social levels into active interaction with one another. It is a
model based on Engel’s original biopsychosocial model (Engel 1977) for which he argued
that to develop a scientific and comprehensive description of mental health, theories that
promote biological reductionism should be dismissed in favour of those that adhere to
general systems theory. The contemporary model, adapted for addiction, reflects an
interactive dynamic for understanding substance use problems specifically and addressing
the complexity of addiction-related issues. The empirical foundation of this model is thus
interdisciplinary, and both descriptive and applied.
The biopsychosocial systems model is grounded in systems theory in which knowledge
occurs at the intersection of the subjective and the objective, and not as an independent
reality. This is a radical departure from the traditional positivist epistemology, which relies
on empirical study and material proof (Bunge 1979; Heylighen, Cilliers, and Gerschenson
2007). Such new iterations of systems theory concentrate on the cognitive and social
processes wherein the construction of subjective knowledge occurs. Each element of a
system may be a complex system in its own right. The dynamic within these relationships
can contribute to or inhibit the emergence of a complex behaviour such as problematic
substance use, while regulating both inputs and outputs from changing internal and external
environments. The complex behaviour contributes both positive and negative feedback, and
thus affects how the complex behaviour emerges. Systems theory, therefore, balances
reductionism and the intrinsic heterogeneity within systems.
The actual social component of the biopsychosocial model is limited, however. The social
domain tends to account only for proximal environmental and social properties. The social
does not necessarily include macrosocial circumstances, such as governmental social
policies, drug policy or drug ‘strategy’ that has a direct effect on substance use rates and
patterns. A systems approach strives to achieve a unification of disciplines neuroscience,
biology, psychology, sociology, philosophy, economics, politics and law by examining
interacting and emerging patterns from each discipline, rather than focusing on common
material components (Heylighen et al. 2007). In this light, the addition of systems to the
prototype biopsychosocial model allows for the inclusion of macrosocial systems as well as
smaller components, such as cells and genes. Together they shape individual actions and
behaviours. A systems approach allows for the inclusion of psycho-social and socially
systemic explanations of addiction, which extend well beyond neurobiology while still
interacting with it (Bunge 1991).
The biopsychosocial systems model implicitly calls for an integrative discussion in the
ethics debate on substance use, decision-making, and responsibility. The model avoids a
forced choice between brain disease and condition of a weak will, and thus provides a useful
framework for overcoming a neuro-essentialist trap. Instead of focusing entirely on causal,
Buchman et al. Page 7
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

reductive neurobiology and difficulties in decision-making, the biopsychosocial systems
model places the individual in his or her social environment and integrates his or her life
narrative. The model contextualizes the responsibility placed on the individual and further
allows for individual members of society to reflect on their own contributions in facilitating
substance misuse (Levy 2007b). The model, therefore, allows for diverse and
multidimensional aspects of knowledge to be drawn upon depending on the concern to be
addressed, and the tools available to address them (Cochrane 2007).
A biopsychosocial systems approach does not portray people as only controlled by the state
of their brains. Addictive behaviours are neither viewed as controlled or uncontrolled but as
difficult to control a matter of degree. Further, the clinically observed defining feature of
addiction a loss of control is understood as a socially normative notion. Thus the claim that
“an addict cannot be a fully free autonomous agent” (Caplan 2008, p.1919) is debatable.
While it assumes that people are usually fully free and autonomous, contemporary
discussions about autonomy as a hyper-value or hypergood (Frank 2004; Gaylin and
Jennings 2003; Taylor 1989) and conceptions of relational autonomy (Sherwin 1998)
necessitate a contextualized discussion of autonomy in debates about addictions (Buchman
and Russell 2009). Because of a tendency to focus on extreme pathological states, the wide
range of normal is often forgotten.
Heroin-Assisted Treatment: An Applied Case Example
Advances in addiction research are increasingly being applied to gain deeper knowledge
about the impact of drug use on brain structure and functioning, capacity, autonomy, free
choice and decision-making, behaviour, treatment, and symptom reduction. While research
of this kind raises important issues about identity, and notions of health and illness, the
outcomes have implications for drug policy, health care systems and delivery, and treatment
for substance use problems.
Addictions research using heroin-assisted treatment (HAT) trials such as the North
American Opiate Medication Initiative (NAOMI) and similar HAT studies and programs in
Europe are a striking, if not controversial example of an effort to embody a biopsychosocial
systems approach. The objective of these trials is to investigate the benefits and risks of
administering medically supervised, pharmaceutical-grade injectable heroin to chronic
opiate users where other treatment options, such as methadone maintenance therapy, have
failed.
The NAOMI trial raised significant scientific, legal, ethical and political concerns, which
included issues of patient safety, the controversial use of placebo control therapy, lack of
equipoise, treatment discontinuation, and compassionate access to treatment (Oviedo-
Joekes, Nosyk, Marsh, et al. 2009). Reflecting on these concerns, the authors stated “we
[had] to be clear in our ethics applications and in our informed consent process with
participants that HAT will not be available outside the context of the study” (p. 267).
Although a full discussion is warranted pertaining to these challenges, these ethical concerns
raised by Oviedo-Joekes et al. (2009) resonate with our present discussion.
Properties of the biopsychosocial systems model are reflected in the case example of HAT.
Here, we examine some of the ethical challenges to research, service delivery, the
philosophies and strategies of harm reduction, and clinical practice that HAT presents.
Integrating Cells and Society
Semi-synthetic opiates such as heroin mainly activate mu opioid receptors in the central
nervous system (Koob, Sanna, and Bloom 1998). Mu receptors activate analgesia,
Buchman et al. Page 8
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

respiratory depression, miosis, euphoria, and reduced gastrointestinal motility. Frequent and
chronic opioid exposure may lead to a significant amount of neuroadaptations, which are
believed to contribute to tolerance, withdrawal, and other mechanisms contributing to the
cycle of compulsive use and relapse (Christie 2008).
Heroin is lipid soluble, which leads to fast penetration of the blood-brain barrier and high
abuse potential (Julien 2001). The reinforcing and euphoric properties of opiates arise from
increased amounts of extracellular dopamine in the ventral tegmental area and nucleus
accumbens. Individuals experiencing withdrawal may suffer severe symptoms that include
sweating, nausea, vomiting, abdominal pain and irritability (Koob and Le Moal 2005). The
risk of mortality is increased due to overdoses; there is an increased risk of acquiring
bacterial infections, and other blood-borne pathogens such as HIV and HCV, as described
earlier. Concurrent mental illness and addiction the norm rather than exception further
characterize individuals with severe opiate addiction (Rush, Urbanoski, Bassani, et al.
2008).
Chronic opioid dependence is associated with a high prevalence of health and psychosocial
issues (Oviedo-Joekes et al 2009), such as high rates of underemployment and
unemployment, involvement with the legal system, unstable housing, and street-involvement
(Fischer, Rehm, Brissette, et al 2005). Research from Europe suggests that chronic heroin-
injection users, who received heroin under supervision in tandem with drug treatment, were
less likely to be involved in criminal activities (Killias, Aebi, and Ribeaud 1998), had better
treatment retention (Haasen, Verthein, Degkwitz et al. 2007), improved social functioning,
and had reduced mortality (Rehm and Fischer 2008). Data from Canadian trials (Oviedo-
Joekes, Nosyk, Brissette et al., 2008), show a greater percentage of female participants
compared to the European trials and a high percentage more than 70% of participants
residing in precarious housing.
Additionally, a recent study by Lasnier, Brochu, Boyd, and Fischer (2009) did not find any
evidence of an increase or decrease in community-based crime associated with HAT clinics
in Canada.
The deontological principle of respect for persons is a characteristic feature of harm
reduction efforts such as HAT. This ethical principle is justified and framed as a matter of
human rights, which maintains that injection drug users, for example, have the right, like
other less stigmatized members of society, to access medical and social services. This claim
coincides with a recent emergence of a global advocacy movement that seeks to construct
the use of drugs as a human right (Elliott, Csete, Wood, and Kerr 2005; Lines and Elliott
2007).
Hunt (2004) takes the rights-based notion further and identifies and characterizes two ethics
of harm reduction. First, he describes a “weak” rights ethic, wherein individuals have the
right to access good healthcare. Second, Hunt identifies a “strong” rights account that
acknowledges a basic right to use drugs. Based on this definition, we believe that HAT falls
into both camps HAT seeks to promote the right to access good health care, and the basic
right as an individual asserting sovereignty over his or her body to inject heroin.
Stigma, Heroin Assisted Treatment, and the Biopsychosocial Systems Model
Whether or not drug use is recognized or constructed as a basic right, individuals living with
an addiction are amongst the most stigmatized members of society. Although stigma is
entrenched in all levels of social fabric, including health care services, it is important to
understand how individuals who participate in clinical trials or treatment may perceive the
supervised injecting of heroin. In a recent study examining stigma and supervised
Buchman et al. Page 9
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

methadone maintenance therapy, Anstice, Strike, and Brands (2009) found that convenient
access to services, relationships with pharmacists and dispensing staff, and characteristics of
where the methadone was distributed were intimately linked with the client’s experiences of
being stigmatized. Moreover, the authors noted that some methadone dispensing locations
helped clients mitigate a stigmatized identity of “a drug user”; while other dispensing
locations exposed or perhaps did not take adequate steps to limit or minimize this effect.
Building on the findings from Anstice et al., future studies can examine ways in which
treatment outcomes can be improved by reducing stigma associated with supervised
treatment such as HAT.
The social burden of illicit drug addiction is estimated at billions of dollars per year (Fisher,
Oviedo-Joekes, Blanken, et al. 2007). Research that involves providing drugs to individuals
living with an addiction must negotiate between science, ethics, politics, law, and evidence-
based medicine. For instance, despite its cost-effectiveness and ease on burden of disease,
the supervised injection site (SIS) in the Downtown Eastside area of Vancouver, Canada has
been repeatedly threatened with closure by politicians. The threats are based on emotional
and moral attitudes towards the existence of the SIS and drug addicts generally, as opposed
to empirical evidence (Des Jarlais, Arasteh, and Hagan 2008).
The structural systems of society have a significant effect not only on the provision of
service delivery, but also on the design of randomized controlled trials like NAOMI, which
seek to minimize the health and social burden of drug addiction. Social justice demands that
politicians permit, and researchers and health care providers scientifically evaluate, more
controversial methods to treat stigmatized conditions such as addiction, where other
methods have either failed or provided limited benefit for the most marginalized members of
society. Accordingly, Oviedo-Joekes et al (2009) describe the systems approach to heroin-
assisted treatment:
“HAT is not simply a pharmacotherapy; it is a treatment approach that is situated
within a context involving neighborhood factors, the local drug scene, housing,
policing, medical care, and other treatment services. Its role and effectiveness is
entangled with the ancillary services available, drug policies, and treatment
philosophy” (p.262).
The biopsychosocial systems approach provides the impetus for a benevolent view of
individuals who have a serious addiction, such as heroin, and the data to date suggest that,
however unorthodox, the intervention appropriately address addiction related issues,
including stigma, at both the individual and societal level.
Conclusions
Robert K. Merton observed that, “In the modern world, the visibly practical
accomplishments of a science largely affect the social value placed upon it” (Merton 1961,
697). Media headlines such as “Brain’s Addiction Centre Found” (BBC 2007) speak to the
power of neuroscience and its ability to construct images of the brain, such that it has
become easy to defer to its account of the complex phenomena that constitute addiction.
Neuroethics challenges arise when knowledge exclusively from neuroscience is deemed
adequate to obtain a full understanding of a mental health disorder as complex as addiction.
While the practicality of biopsychosocial systems model may allow for a more integrative
explanation for addiction, it does not explain addiction entirely. Indeed, there is no single
theory or approach that can offer a complete explanation for the existence of any social
problem (Merton 1961). Moreover, the model does not solve the problem of free choice, as
the model still, even at the systems (macro) level, has causally sufficient preceding
conditions.
Buchman et al. Page 10
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

It is the integration of biological data and psycho-social, narrative, family information, and
clinical phenomenology that will make way for more precise forecasting and earlier
diagnosis than is possible today. This is one path to follow for new opportunities for
treatment and intervention directed toward prevention. Accordingly, an analysis of the
ethical, legal and social issues around other problems of addiction, such as prescription
opiate misuse for pain management, may also be examined within the context of our
proposed framework.
The application of a multi-dimensional model like the model proposed here is not
revolutionary. As a rule, mental health workers are familiar with an integrative
understanding of addiction, and would not recommend a treatment intervention based on
biological information alone. However the rapid developments in neuroscience are moving
bio-psychiatry away from the mind, and towards actions in the brain. Mind once was the
place of mediation between person and situation, between the biological and the social. How
these advances will impact the ethical relationship between our brains and our selves in
addiction, is yet to be seen.
Acknowledgments
This paper is based on a thesis in fulfillment of the requirements for a Master of Social Work in the Collaborative
Program in Addiction Studies at the University of Toronto. Special thanks to Dr. Barbara Russell and Dr. Marilyn
Herie at both the Centre for Addiction and Mental Health and the University of Toronto who advised on earlier
versions of the manuscript; Neil Chahal and Sofia Lombera at the National Core for Neuroethics at the University
of British Columbia; the two anonymous reviewers for their thoughtful remarks. Supported by NIH/NIMH (#MH
R01MH84282-04A1), the Canadian Institutes for Health Research (CNE #85117), the Michael Smith Foundation
for Health Research, and the Vancouver Coastal Health Research Institute.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR
fourth edition text revision. Washington, DC: American Psychiatric Association; 2004.
Andreou C. Making a clean break: Addiction and Ulysses contracts. Bioethics 2008;22:25–31.
[PubMed: 18154586]
Anstice S, Strike CJ, Brands B. Supervised methadone consumption: Client issues and stigma.
Substance Use and Misuse 2009;44(6):794–808. [PubMed: 19444722]
Irwin, T., translator. Aristotle. Nicomachean ethics. 2. Indianapolis: Hackett Publishing Company, Inc;
1999.
Ashcroft, R.; Campbell, AV.; Capps, B. Ethical aspects of developments in neuroscience and drug
addiction. In: Nutt, D.; Robbins, TW.; Stimson, GV.; Ince, M.; Jackson, A., editors. Drugs and the
future. London: Elselvier; 2007. p. 439-465.
Babor, T.; Caetano, R.; Casswell, S.; Edwards, G.; Giesbrecht, N.; Graham, K. Alcohol: No ordinary
commodity: Research and public policy. New York: Oxford University Press; 2003.
BBC. Brain’s ‘addiction centre’ found. Jan Thursday 25. 2007 Retrieved 3 December 2008, from
http://news.bbc.co.uk/2/hi/health/6298557.stm
Bechara A, Damasio H. Decision-making and addiction (part1): impaired activation of the somatic
states in substance dependent individuals when pondering decisions with negative future
consequences. Neuropsychologia 2002;40:1675–1689. [PubMed: 11992656]
Buchman DZ, Russell BJ. Addictions, autonomy and so much more: A reply to Caplan. Addiction
2009;104:1053–1055. [PubMed: 19466928]
Bunge, M. Treatise on basic philosophy Vol. 4: A world of systems. Dordrecht: Kluwer; 1979.
Bunge, M. The power and limits of reduction. In: Agazzi, E., editor. The problem of reductionism in
science. Dordrecht: Kluwer Academic Publishers; 1991. p. 31-49.
Bunge M. Mechanism and explanation. Philosophy of the Social Sciences 1997;27:410–465.
Bunge, M. Emergence and convergence. Toronto: University of Toronto Press; 2003.
Buchman et al. Page 11
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

Bunge M. How does it work? The search for explanatory mechanisms. Philosophy of the Social
Sciences 2004;34:182–210.
Burns K, Bechara A. Decision making and free will: A neuroscience perspective. Behavioral Science
and the Law 2007;25:263–280.
Caetano R, Clark C. Trends in alcohol consumption patterns among Whites, Blacks and Hispanics:
1984 and 1995. Journal of Studies on Alcohol 1998;59:659–668. [PubMed: 9811087]
Caplan AL. Ethical issues surrounding forced, mandated or coerced treatment. Journal of Substance
Abuse Treatment 2006;31:117–120. [PubMed: 16919736]
Caplan AL. Denying autonomy in order to create it: The paradox of forcing treatment upon addicts.
Addiction 2008;103:1919–1921. [PubMed: 19469727]
Carter A, Hall W. Informed consent to opioid agonist maintenance treatment: Recommended ethical
guidelines. International Journal of Drug Policy 2008a;19(1):79–89. [PubMed: 18077146]
Carter A, Hall W. The issue of consent in research that administers drugs of addiction to addicted
persons. Accountability in Research 2008b;15(4):209–225. [PubMed: 18972263]
Charland LC. Cynthia’s dilemma: Consenting to heroin prescription. The American Journal of
Bioethics 2002;2(2):37–47. [PubMed: 12189075]
Charland LC. Heroin addicts and consent to heroin therapy: A comment on Hall et al. Addiction
2003;98(11):1634–1635. [PubMed: 14616191]
Childress AR, Mozely PD, McElgin W, Fitzgerald J, Reivich M, O’Brian CP. Limbic activation during
cue-induced cocaine craving. American Journal of Psychiatry 1999;156:11–18. [PubMed:
9892292]
Christie MJ. Cellular neuroadaptations to chronic opioids: Tolerance, withdrawal and addiction.
British Journal of Pharmacology 2008;154(2):384–396. [PubMed: 18414400]
Christman J. Relational autonomy, liberal individualism, and the social constitution of selves.
Philosophical Studies 2004;117:143–164.
Cochrane TI. Brain disease or moral condition? Wrong question. American Journal of Bioethics
(AJOB-Neuroscience) 2007;7(1):24–25.
Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nature
Neuroscience 2005;8(11):1431–1436.
Damasio, AR. Descartes’ error. New York: Harper Collins; 1994.
Dehue T. A Dutch treat: Randomized controlled experimentation and the case of heroin-maintenance
in the Netherlands. History of the Human Sciences 2002;15(2):75–98.
Des Jarlais DC, Kamyar A, Hagan H. Evaluating Vancouver’s supervised injection facility: Data and
dollars, symbols and ethics. Canadian Medical Association Journal 2008;179(11):1105–1106.4.
[PubMed: 19015552]
Dietrich S, Matschinger H, Angermeyer MC. The relationship between biogenetic causal explanations
and social distance toward people with mental disorders: Results from a population survey in
Germany. The International Journal of Social Psychiatry 2006;52(2):166–174. [PubMed:
16615248]
Dom G, Sabbe B, Hulstijn W, Van Den Brink W. Substance use disorders and the orbitofrontal cortex.
British Journal of Psychiatry 2005;187:209–220. [PubMed: 16135857]
Dumit J. Is it me or my brain? Depression and neuroscientific facts. Journal of Medical Humanities
2003;24(1–2):35–47.
Elliott R, Csete J, Wood E, Kerr T. Harm reduction, HIV/AIDS, and the human rights challenge to
global drug control policy. Health and Human Rights 2005;8:104–138. [PubMed: 17136905]
Elster, J.; Skog, O-J. Getting hooked: Rationality and addiction. Cambridge, U.K: Cambridge
University Press; 1999.
Engel GL. The need for a new medical model: A challenge for biomedicine. Science 1977;196:129–
136. [PubMed: 847460]
Fischer B, Rehm J, Brissette S, Brochu S, Bruneau J, El-Guebaly, et al. Illicit opioid use in Canada:
Comparing social, health, and drug use characteristics of untreated users in five cities (OPICAN
study). Journal of Urban Health 2005;82(2):250–266. [PubMed: 15872194]
Buchman et al. Page 12
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

Fischer B, Oviedo-Joekes E, Blanken P, Haasen C, Rehm J, Schechter MT, Strang J, van den Brink W.
Heroin-assisted treatment (HAT) a decade later: A brief update on science and politics. Journal of
Urban Health 2007;84(4):552–562. [PubMed: 17562183]
Foddy B, Savulescu J. Addiction and autonomy: Can addicted people consent to the prescription of
their drug of addiction? Bioethics 2006;20:1–15. [PubMed: 16680876]
Foddy B, Savulescu J. Addiction is not an affliction: Addictive desires are merely pleasure-oriented
desires. The American Journal of Bioethics (AJOBNeuroscience) 2007;7:29–32.
Frank A. Ethics as process and practice. Internal Medicine Journal 2004;34:355–357. [PubMed:
15228397]
Fry, CL. A social neuroethics of addiction: Revealing public and private identities and self
understanding. Poster at the 1st Annual Meeting of the Neuroethics Society; Washington, D.C.,
USA. 2008.
Gaylin, W.; Jennings, B. The perversion of autonomy: Coercion and constraints in a liberal society.
Washington: Georgetown University Press; 2003.
Gillett G. Autonomy and selfishness. The Lancet 2008a;372(9645):1214–1215.
Gillett, G. Subjectivity and being somebody: Human identity and neuroethics. Exter: Imprit
Academics; 2008b.
Gillett G. Intention, autonomy, and brain events. Bioethics 2009;23(6):330–339. [PubMed: 19527261]
Godin G, Kok G. The theory of planned behavior: A review of its applications to health-related
behaviors. American Journal of Health Promotion 1996;11:87–98. [PubMed: 10163601]
Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid
dependence. British Journal of Psychiatry 2007;191:55–62. [PubMed: 17602126]
Haggard P. Human volition: Towards a neuroscience of will. Nature Reviews Neuroscience
2008;9:934–946.
Hall W, Capps B, Carter A. The use of depot naltrexone under legal coercion: The case for caution.
Addiction 2008;103:1922–1924. [PubMed: 19469728]
Hall W, Carter L, Morely KI. Addiction, neuroscience and ethics. Addiction 2003a;98(7):867–870.
[PubMed: 12814489]
Hall W, Carter L, Morely KI. Addiction, ethics and scientific freedom. Addiction 2003b;103:873–874.
[PubMed: 18482408]
Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems
in adolescence and early adulthood: Implications for substance abuse prevention. Psychological
Bulletin 1992;112:64–105. [PubMed: 1529040]
Health Canada. Canadian tobacco use monitoring survey. 2007. August 25, 2008Retrieved December
5, 2008, from
http://www.hc-sc.gc.ca/hl-vs/tobac-tabac/researchrecherche/stat/ctums-esutc_2007-eng.php
Heylighen, F.; Cilliers, P.; Gerschenson, C. Complexity and philosophy. In: Bogg, J.; Geyer, R.,
editors. Complexity, science and society. Radcliffe: Oxford University Press; 2007.
Hunt N. Public health or human rights: What comes first? International Journal of Drug Policy
2004;15(4):231–237.
Hyman SE. Addiction: a disease of learning and memory. The American Journal of Psychiatry
2005;162(8):1414–1422. [PubMed: 16055762]
Hyman SE. The neurobiology of addiction: Implications for voluntary control of behavior. The
American Journal of Bioethics (AJOB-Neuroscience) 2007;7(1):8–11.
Julien, RM. A primer of drug action. 9. New York: Worth Publishers; 2001.
Kaufman J, Ross T, Stein E, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO
task as revealed by event-related functional magnetic resonance imaging. The Journal of
Neuroscience 2003;23:7839–7843. [PubMed: 12944513]
Killias M, Aebi M, Ribeaud D. Effects of heroin prescription on police contacts among drug-addicts.
European Journal on Criminal Policy and Research 1998;6(3):433–438.
Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature
Neuroscience 2005;8:1442–1444.
Buchman et al. Page 13
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron 1998;21(3):467–476. [PubMed:
9768834]
Lasnier B, Brochu S, Boyd N, Fischer B. A heroin prescription trial: Case studies from Montreal and
Vancouver on crime and disorder in the surrounding neighbourhoods. International Journal of
Drug Policy. 2009 in press.
Lauer, RH.; Lauer, JC. Social problems and the quality of life. 8. New York: McGraw Hill Higher
Education; 2002.
Leshner AI. Addiction is a brain disease, and it matters. Science 1997;278:45–57. [PubMed: 9311924]
Levy N. Autonomy and addiction. Canadian Journal of Philosophy 2006;36(3):427–448.
Levy, N. Neuroethics: Challenges for the 21st century. New York: Cambridge University Press;
2007a.
Levy N. The social: A missing term in the debate over addiction and voluntary control. American
Journal of Bioethics 2007b;7:35–36. [PubMed: 17366163]
Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-
limbic dysfunction in psycho-stimulant addiction. Neuroscience and Biobehavioral Reviews
2008;32:581–597. [PubMed: 18164058]
Lines R, Elliott R. Injecting drugs into human rights advocacy. International Journal of Drug Policy
2007;18:453–457. [PubMed: 17703935]
Loewenstein G. Willpower: A decision theorist’s perspective. Law and Philosophy 2000;19:51–76.
Mehta S, Farina A. Is being sick really better? Effect of the disease view of mental disorder on stigma.
Journal of Social and Clinical Psychology 1997;16(4):405–419.
Merton, RK. Social problems and sociological theory. In: Merton, RK.; Nisbet, RA., editors.
Contemporary Social Problems. New York: Harcourt; 1961. p. 697-737.
Miller, WR.; Carroll, KM. Drawing the science together: Ten principles, ten recommendations. In:
Miller, WR.; Carroll, KM., editors. Rethinking substance abuse: What the science shows, and what
we should do about it. New York: The Guilford Press; 2006. p. 293-311.
Morse SJ. Voluntary control of behavior and responsibility. The American Journal of Bioethics
2007;7(1):12–36. [PubMed: 17366152]
Oviedo-Joekes E, Nosyk B, Brissette S, Chettiar J, Schneeberger P, Marsh DC, et al. The North
American opiate medication initiative (NAOMI): Profile of participants in North America’s first
trial of heroin-assisted treatment. Journal of Urban Health 2008;85(6):812–825. [PubMed:
18758964]
Oviedo-Joekes E, Nosyk B, Marsh DC, Guh D, Brissette S, Gartry C, et al. Scientific and political
challenges in North America’s first randomized controlled trial of heroin assisted treatment for
severe heroin addiction: Rationale and design of the NAOMI study. Clinical Trials 2009;6(3):261–
271. [PubMed: 19528135]
Phelan J, Yang L, Cruz-Rojas R. Effects of attributing serious mental illnesses to genetic causes on
orientations to treatment. Psychiatric Services 2006;57:382–387. [PubMed: 16524997]
Racine E, Bar-Ilan O, Illes J. fMRI in the public eye. Nature Reviews Neuroscience 2005;6(2):159–
164.
Raphael, D. Introduction to the social determinants of health. In: Raphael, D., editor. Social
determinants of health: Canadian perspectives. Toronto: Canadian Scholars’ Press Inc; 2004.
Read J. Why promoting biological ideology increases prejudice against people labelled
“schizophrenic”. Australian Psychologist 2007;42(2):118–128.
Read J, Haslam N, Sayce L, Davies E. Prejudice and schizophrenia: A review of the ‘mental illness is
an illness like any other’ approach. Acta Psychiatrica Scandinavica 2006;114:303–318. [PubMed:
17022790]
Rehm J, Fischer B. Should heroin be prescribed to heroin misusers? Yes. British Medical Journal
2008;336(7635):70. [PubMed: 18187720]
Reinarman C, Cohen PD, Kaal HL. The limited relevance of drug policy: Cannabis in Amsterdam and
in San Francisco. American Journal of Public Health 2004;94:836–842. [PubMed: 15117709]
Buchman et al. Page 14
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript

Rush B, Urbanoski K, Bassani D, Castel S, Wild TC, Strike C, Kimberly D, Somers J. Prevalence of
co-occurring substance use and other mental disorders in the Canadian population. Canadian
Journal of Psychiatry 2008;53(12):800–809.
Russell B. Patient autonomy writ large. The American Journal of Bioethics 2009;9(2):32–34.
[PubMed: 19180391]
Satel SK. What should we expect from drug abusers? Psychiatric Services 1999;50:861. [PubMed:
10402604]
Searle, JR. Freedom and neurobiology. New York: Columbia University Press; 2004.
Sherwin, S. A relational approach to autonomy in health care. In: Sherwin, S., editor. The Politics of
Women’s Health: Exploring agency and autonomy. Philadelphia: Temple University Press; 1998.
p. 19-47.
Shiloh S, Rashuk-Rosenthal D, Benyamini Y. Illness causal attributions. An exploratory study of their
structure and associations with other illness cognitions and perceptions of control. Journal of
Behavioral Medicine 2002;25:373–394. [PubMed: 12136498]
Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse
and clinical implications. Drug and Alcohol Review 2007;26:25–31. [PubMed: 17364833]
Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human
brain. Nature Neuroscience 2008;11(5):543–545.
Strike CJ, Myers T, Millson M. Finding a place for needle exchange programs. Critical Public Health
2004;14(3):261–275.
Taylor, C. Sources of the self: The making of modern identity. Cambridge: Harvard University Press;
1989.
Thomas YF. The social epidemiology of drug abuse. American Journal of Preventive Medicine
2007;32(6S):S141–S146.
Uhl G, Drgon T, Johnson C, Liu Q-R. Addiction genetics and pleiotropic effects of common
haplotypes that make polygenic contributions to vulnerability to substance dependence. Journal of
Neurogenetics. 2009 [Epub ahead of Print].
Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology
2009;56:48–62. [PubMed: 18722390]
Volkow N, Li TK. The neuroscience of addiction. Nature Neuroscience 2005;8:1429–1430.
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and
addiction. Neuropharmacology 2009;56:3–8. [PubMed: 18617195]
Wallace JJ. The social ecology of addiction: Race, risk and resilience. Pediatrics 1999;103:1122–1127.
[PubMed: 10224199]
Wallace JJ, Bachman J, O’Malley P, Johnston L, Schulenberg J, Cooper S. Tobacco, alcohol, and illict
drug use: Racial and ethnic differences among U.S. high school seniors, 1976–2000. Public
Health Reports 2002;117(suppl 1):S67–S75. [PubMed: 12435829]
Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology
2005;5:9–19. [PubMed: 15661620]
Wegner, D. The illusion of conscious will. Cambridge: MIT Press; 2002.
Xu Z, Hou B, Gao Y, He F, Zhang C. Effects of enriched environment on morphine-induced reward in
mice. Experimental Neurology 2007;204(2):714–719. [PubMed: 17331503]
Buchman et al. Page 15
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript
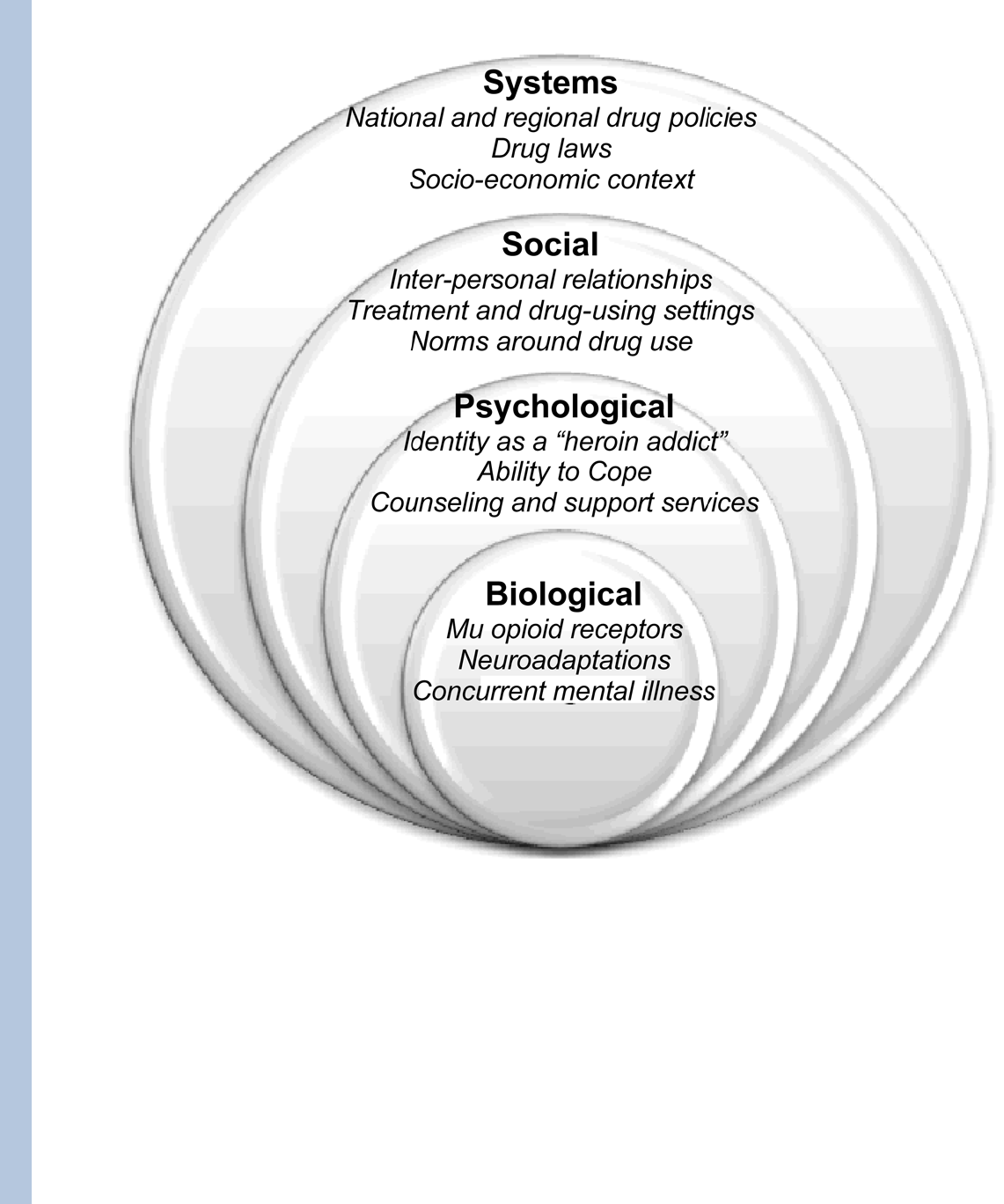
Figure 1.
The Biopsychosocial Systems Model of Addiction. Primary features of the model are shown
in boldface; variables exemplifying heroin-assisted treatment are shown in italics.
Buchman et al. Page 16
AJOB Neurosci. Author manuscript; available in PMC 2011 January 1.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript
